Heartburn Health Check with EndoSign®


The EndoSign® Barrett's Esophagus Test is a simple and accurate test that can detect signs of precancer to protect your patients’ esophageal health.
Why is diagnosing Barrett’s esophagus important?
Esophageal adenocarcinoma (EAC) accounts for the majority of esophageal cancers in the U.S. and is on the rise 1,2
<20%
survive 5 years from diagnosis3
Prognosis is strongly linked to stage of diagnosis, which is why early detection is so important
5.6%
diagnosed at late stage survive 5 years4
EAC arises from Barrett’s esophagus, where cells lining the esophagus undergo metaplastic replacement
10X
Increased risk of developing EAC with Barrett’s vs without 5
Identifying patients at the precancerous stage, Barrett’s esophagus, gives the ability to intervene early with curative therapy options.
What is EndoSign®?
The EndoSign® Barrett's Esophagus Test is a laboratory developed test that uses next-generation sequencing to identify individuals with Barrett's esophagus. The test analyzes differentially methylated genomic regions in esophageal cell samples collected by the FDA 510(k)-cleared EndoSign® Cell Collection Device.
The EndoSign® Barrett's Esophagus Test can detect precancer in individuals before they develop symptoms of cancer.

Who is it for?
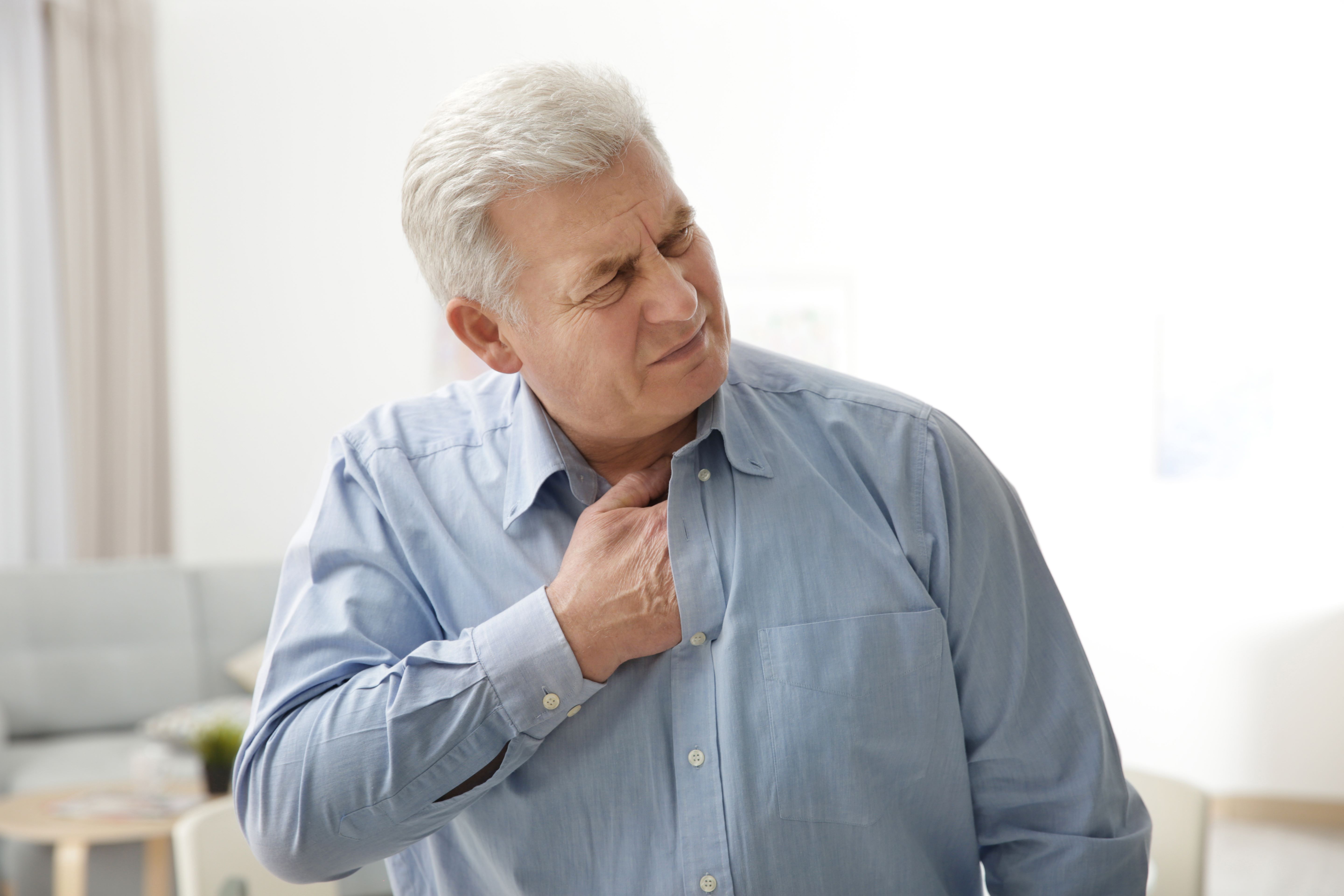
Age and chronic GERD are the greatest risk factors for Barrett’s. The American College of Gastroenterologists and American Gastroenterology Association recommend a swallowable, non-endoscopic cell collection device, combined with a biomarker analysis, to identify Barrett’s in patients with at least three established risk factors:1,2
Heartburn or reflux is the best early indicator for Barrett’s and esophageal cancer.8-10
Smoking or a history of smoking puts you at a greater risk of developing these conditions.12
Men are twice as likely to be diagnosed with esophageal cancer and Barrett’s compared to women.11
Non-hispanic white people are at a higher risk of developing these conditions, but all groups can be affected.13
There is an age-associated risk for these conditions in people aged over 50.6
Your likelihood of having Barrett’s increases if you have a family history of Barrett’s or esophageal cancer.14
Body fat around your abdomen further increases your risk.6-8
How it works
The EndoSign® Barrett's Esophagus Test uses a simple non-endoscopic capsule sponge procedure to collect pan-esophageal cell samples for analysis.
Request the test
You should determine if the test is suitable for your patient based on their symptoms and medical history.
Collect sample
The procedure can be completed in less than 10 minutes and there is no need for your patient to be sedated.

Test
The test analyzes esophageal samples for methylation biomarkers specific to Barrett’s esophagus.
Results
A comprehensive, easy-to-interpret diagnostic report will be sent to you to review with your patient.
Getting started with EndoSign®
The EndoSign® Barrett's Esophagus Test is intended to provide a quantitative estimate of the likelihood that an EndoSign® capsule sponge sample is representative of a patient with Barrett’s esophagus using a limited set of DNA methylation targets.
Intended Patient Population: Patients considered to be at a high risk of Barrett’s esophagus (BE)/esophageal adenocarcinoma (EAC) based on the risk factors listed by the AGA/ACG (PMID:35788412), specifically individuals with at least 3 established risk factors including individuals who are male, non-Hispanic white, age >50 years, have a history of smoking, chronic gastroesophageal reflux disease (GERD), obesity, or a family history of BE or EAC.
Results should be interpreted by a healthcare provider in the context of medical history, clinical signs and symptoms. A test result of “No Barrett’s Detected” does not rule out Barrett’s esophagus. A test result of “Barrett’s Signal Detected” requires confirmatory diagnostic evaluation by endoscopy to confirm. False-positive and false-negative test results do occur. Rx only.
Cyted Health’s clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA). The EndoSign® Barrett's Esophagus Test was developed, and its performance characteristics were determined by Cyted Health. The EndoSign® Barrett's Esophagus Test has not been cleared or approved by the Food and Drug Administration. Cyted Health’s clinical laboratory is regulated under CLIA to perform high-complexity testing. The EndoSign® Barrett's Esophagus Test is intended for clinical purposes.
Esophageal cell samples are collected with the EndoSign(R) Cell Collection device in a short office-based procedure. A vitamin-sized pill is swallowed and dissolves in the stomach, releasing a small self-expanding sponge in 7 minutes. The sponge collects surface cells along the length of the esophagus, and the sample is placed in a preservation kit and sent to Cyted Health's laboratory for analysis to detect Barrett's Esophagus.
The EndoSign(R) Cell Collection device received 510(k) clearance by the FDA in January 2024, demonstrating that it is safe and effective for use.
- Arnold M, Ferlay J, van Berge Henegouwen MI, et alGlobal burden of oesophageal and gastric cancer by histology and subsite in 2018 Gut 2020;69:1564-1571.
- Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology. 2022 Sep;163(3):649-658.e2. Doi: 10.1053/j.gastro.2022.05.054. Epub 2022 Jun 4. PMID: 35671803.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review (CSR) 1975-2017. SEER. 2020. Accessed March 5, 2024. https://seer.cancer.gov/csr/1975_2017/ .
- American Cancer Society 2024. Cancer Statistics Center. (Online, accessed March 5th 2024)
- Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365(15):1375-1383.
- Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and management of Barrett's esophagus: An updated acg guideline. Am J Gastroenterol. 2022;117(4):559-587.
- Muthusamy VR, Wani S, Gyawali CP, Komanduri S; CGIT Barrett’s Esophagus Consensus Conference Participants. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett's Esophagus: Expert Review. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2696-2706.e1. doi: 10.1016/j.cgh.2022.06.003. Epub 2022 Jul 3. PMID: 35788412; PMCID: PMC10203866.
- Smyth et al. (2017). Oesophageal cancer. Nature reviews. Disease primers, 3, 17048. (Online) Available at: https://pubmed.ncbi.nlm.nih.gov/28748917/
- Cook MB, et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: a pooled analysis from the Barrett's and Esophageal Adenocarcinoma Consortium (BEACON) PLoS One. 2014;9:e103508
- Thrift AP. Barrett's Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Dig Dis Sci. 2018;63(8):1988-96.
- Yousef et al. (2008) The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta analysis. American Journal of Epidemiology. (Online) Available at: https://pubmed.ncbi.nlm.nih.gov/18550563/
- Cook et al. (2012) Cigarette smoking increases risk of Barrett’s esophagus: an analysis of the Barrett’s and Esophageal Adenocarcinoma Consortium. Gastroenterology (Online). Available at: https://pubmed.ncbi.nlm.nih.gov/22245667/
- Corely et al (2009) Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community based study. (Online). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2671084/
- Peters et al (2021) Increased risk of Barrett’s oesophagus and related neoplasia in individuals with a positive family history. European Journal of Cancer (Online) Available at: https://www.sciencedirect.com/science/article/pii/S0959804921004494

